What Type of Bonds Hold Water Molecules Together
Instead of losing electrons hydrogen and oxygen share. Hydrogen and oxygen atoms have different electronegativity values which.
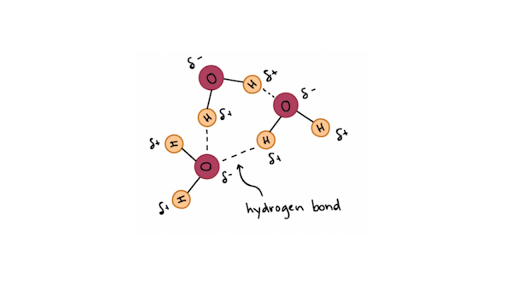
Hydrogen Bonds In Water Article Khan Academy
Which type of chemical bond holds water molecules together with other water molecules.

. In a b the polar covalent bonds are shown as lines. Different ways of representing the polar sharing of electrons in a water molecule. Which type of bonds form between water molecules.
Polar Covalent bonds hold together a water molecule because oxygen is a way more electronegative than hydrogen. Water molecules are made up of two hydrogen atoms and one oxygen atom held together by polar covalent bonds. In part c the polar covalent bonds are shown as electron dots shared by the oxygen and hydrogen atoms.
Water molecules are made up of two hydrogen atoms and one oxygen atom held together by polar covalent bonds. Each diagram shows the unsymmetrical shape of the water molecule. The weak attractive forces that hold water molecules together are called hydrogen bonds Compared to the polar covalent bonds that hold the oxygen and hydrogen atoms together within a molecule of water the hydrogen bonds that hold multiple water molecules together are.
They bonded together through covalent bonding. - Polar covalent bonds - Non-polar covalent bonds - Ionic bonds - Hydrogen bonds. The oxygen region of a water molecule has a slightly negative charge whereas the hydrogen portions of the molecule take on slightly positive charges.
However when water molecules are placed together as they are normally the hydrogen atoms in each molecule can form hydrogen bonds with the oxygen atom of other molecules. Hydrogen Bonds Make Water Sticky. Water molecules have covalent bonds.
Nicolle Rager Fuller National Science Foundation. Hydrogen and oxygen are non-metals. Structure of proteins is determined by hydrogen bonds between amino acids that cause proteinto coil into helices or pleated sheets.
A water molecule consists of two atoms of hydrogen linked by covalent bonds to the same atom of oxygen. Hydrogen bonds allow two molecules to link together temporarily. Water molecules are held together by hydrogen bonds.
Atoms of oxygen are electronegative and attract the shared electrons in their covalent bonds. The polarity of the water molecule is what gives water its unique properties. Consequently the electrons in the water molecule spend slightly more time around the oxygen atomic center and less time around the hydrogen atomic centers.
Each molecule consists of two hydrogen and oxygen covalent bonds. The type of bonding that holds two or more water molecules together is called hydrogen bonding. Water molecules are polar meaning they have slightly negative and positive regions within the molecule.
But there will also be hydrogen bonds formed between adjacent water molecules which accounts for its unusually high boiling point among other things. Hydrogen-bonding forms in liquid water as the hydrogen atoms of one water molecule are then attracted towards the oxygen atom of a neighboring water molecule resulting in a general proton shared. Although short-lived and much weaker than the covalent variety hydrogen bonds contribute significantly to water chemistry because they are extremely abundant in H 2 O.
The property of cohesion describes the ability of water molecules to be attracted to other water molecules which allows water to be a sticky liquid. Chemistry the tendency of an atom or radical to attract electrons in the formation of an ionic bond. Each H 2 O can bind to a maximum of four neighbors through these so-called hydrogen bonds.
Hydrogen bonds are bonds between a hydrogen in one polar molecule and the negatively charged end of another polar molecule. Lacking affinity for water Repelling tending not to combine with or incapable of dissolving in water. Water molecules are made up of two hydrogen atoms and one oxygen atom held together by polar covalent bonds.
It is an intermolecular bonding interaction between two molecules of different electronegative values or bonding between lone pairs of electron rich molecules. Hydrogen bonds are attractions of electrostatic force caused by the difference in charge between slightly. Water molecules forming hydrogen bonds with one another.
Water has an amazing ability to adhere stick to itself and to other substances. A water molecule is held together with polar covalent bonds. Hydrogen bonds are bonds between a hydrogen in one polar molecule and the negatively charged end of another polar moleculeHydrogen bonds allow two molecules to link together temporarily.
The partial negative charge on the O of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other. Having a strong affinity for water. Covalent hydrogen Which type of chemical bond holds water molecules together by forming a bridge between negative oxygen atom of one water molecule positive hydrogen atoms of another water molecule.
The bonds that keep a water molecule together as a molecule are covalent bonds between the oxygen atom and the two hydrogen atoms. Hydrogen bonds allow two molecules to link together temporarily.

Chemical Bonds Bio103 Human Biology

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

Comments
Post a Comment